{{faqs.Name}}
-
{{category.Name}}:
-

| Catalog # | Price | Size/Scale | Note | Qty | Add | |
|---|---|---|---|---|---|---|
| {{item.catalogId}} | {{ item.userPriceBook.unitPrice | currency }} | {{ item.unitPrice | currency }} | {{item.sizeAndScale}} | {{item.note}} |
 {{item.message}}
{{item.message}}
|
|
| checkout view cart | ||||||
(S-trityl-6-mercaptohexyl)-(2-cyanoethyl)-(N,N-diisopropyl)-phosphoramidite
C34H45N2O2PS
576.8
colorless oil
Prior to dilution ensure that all product is at the bottom of the vial. Dilute to the recommended concentration and mix thoroughly in the sealed vial to ensure that all contents are dissolved.
100 µmol/mL
The deprotecting conditions for this amidite will be depend on the types of amidites used for the synthesis of the oligo. If using fast deprotecting amidites deprotect in concentrated NH4OH for 1 hour at 60 °C. If using standard amidites deprotect in concentrated NH4OH for 5 hours at 60 °C.
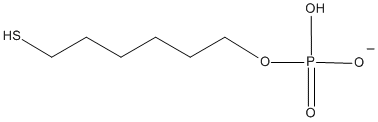
196.21
Cold
-15 to -30 °C
Trityl-Thiohexyl Amidite is used to functionalize the 5' end of an oligonucleotide with a thiol group. Thiol functionalization allows attachment to fluorescent dyes, maleimide compounds or conjugation to proteins through disulfide linkages. The lipophilic trityl group can be used as a handle for RP purification. It cannot be removed using normal acidic deblock methods.

Below is a protocol using reversed-phase MicroPure column (MP-1602) for purification of oligonucleotides. Each MicroPure column will clean up to a 1 µmol synthesis column. (Detailed instructions for the preparation of reagents can be found following the protocol.) 1. Rinse column with CH3CN (2 x 2 mL). 2. Activate column with 1 N TEAA (3 x 2 mL) 3. Add Crude 5'Trityl-Thio 2 mL of 1:1 solution (NH4OH/H2O). (2x) 4. Elute Failures with 3% NH4OH (3 x 2 mL). 5. Rinse column with water (3 x 2 mL). 6. Insert collection tubes; elute purified oligo with 40% MeCN in water. (1 x 1.5 mL) 7. Dry sample in speed vac Preparation of Reagents Five different solutions for the purification process need to be prepared. To store these solutions use clean glass bottles with plastic lined caps. 1. Water. Use good quality sterile, deionized water. HPLC grade is best, especially if subsequent analyses will rely on HPLC methods. 2. Acetonitrile (VWR, cat. number JT9018-3). 3. 40% acetonitrile. Dilute 40 mL of acetonitrile (from #2 above) with 80 mL of water (from #1 above). 4. 1.0 N Triethylammonium acetate (TEAA). For this solution, place about 80 mL of the water (from #1 above) in a clean bottle, and add exactly 5.7 mL of acetic acid (Aldrich cat. number 24, 285-3). Swirl the solution until completely mixed, and add exactly 13.9 mL of triethylamine (Aldrich cat. number 23, 962-3). Mix the solution until it is completely homogeneous - a small stir bar and magnetic stirrer may be used with good results. After complete mixing is achieved, the pH should be neutral. pH paper can be used for this (VWR cat. number 60775-702). (Caution wear eye protection and gloves when using concentrated acetic acid (causes burns) and concentrated triethylamine). 5. 2.5% Trifluoroacetic acid (TFA). Carefully add 2.5 mL of TFA (Aldrich cat. number T6, 220-0) to 97.5 mL of water (from #1 above). Mix the solution until it is completely homogeneous. (Caution - wear eye protection and gloves when using concentrated TFA.) 6. 3.0% Ammonium Hydroxide. Add 10 mL of concentrated ammonia (Aldrich cat. number 22, 122-8) to 90 mL of water (from #1 above). Mix the solution until it is completely homogeneous. (Caution - wear eye protection and gloves when using concentrated ammonia)

196.21