Partner with LGC Biosearch Technologies for quality, consistent custom oligo synthesis throughout your development pipeline. Our experts support the smooth transition of your in vitro diagnostic test (IVD test) from early concept to commercial use.
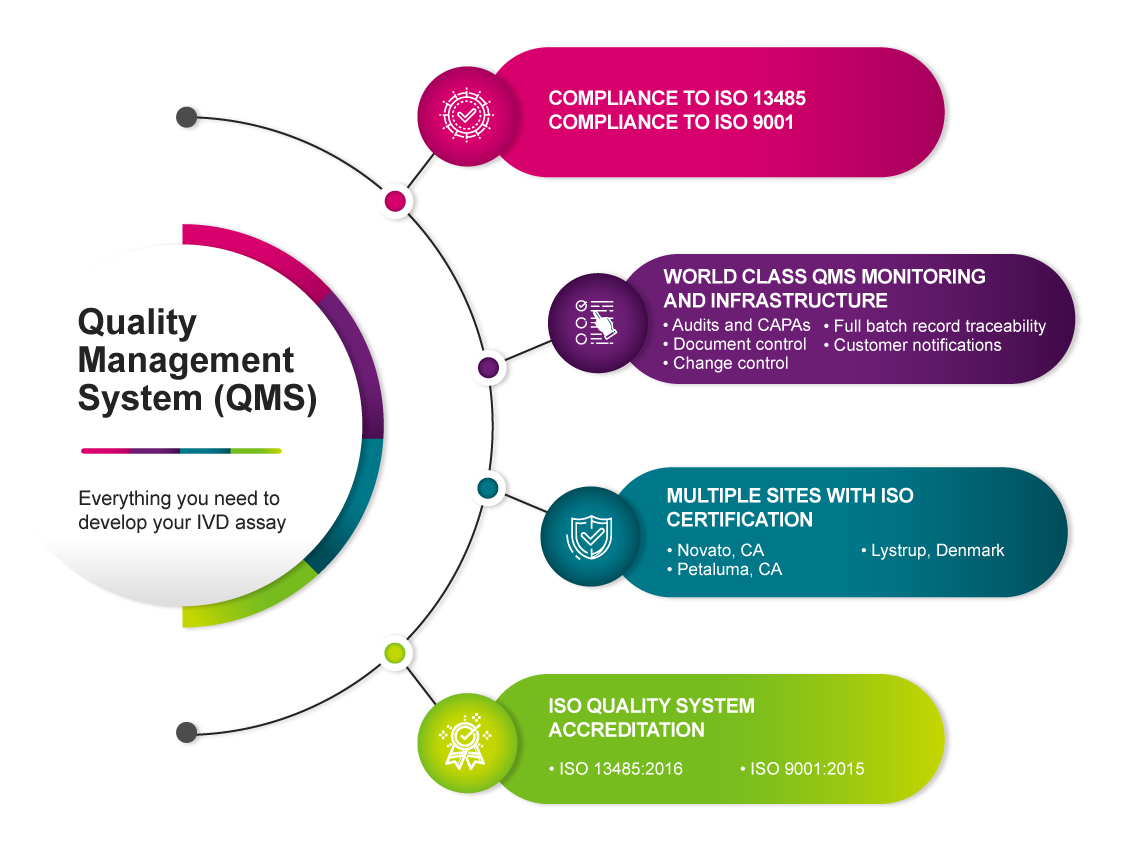
Our commercial grade oligos are manufactured under a quality management system that is ISO 13485-certified for all applicable medical device regulations. Expect quality and reliability across an extensive menu of oligo options:
- Thousands of oligonucleotide modifications, including specialty reagents
- Fluorophores and quenchers spanning your full spectrum of needs
- Customisable oligos fine-tuned to your specifications
Our multi-site global oligonucleotide manufacturing operations feature state-of-the-art equipment and operate under controlled manufacturing processes that ensure lot-to-lot consistency and extremely low risk of contamination. Batch records provide peace of mind and full traceability of manufacturing standards meeting your requirements.
Custom commercial grade oligos for molecular diagnostics
Whether you’re composing a multiplexed assay or pursuing a single target gene, you can rely on the performance of oligos from Biosearch Technologies. We deliver the same quality and consistency as your molecular diagnostics assay advances and scales from nanomoles to millimoles of material.
Why choose LGC Biosearch Technologies for commercial grade oligos:
- Scalable and consistent performance: By leveraging our experience in scale-up and lean operations, we enable rapid product development from concept to validation and commercialisation, while maintaining consistency and high quality from small to large scale production.
- High production standards: We’re one of the only manufacturers in the world that can take you from R&D to IVD to therapeutic-grade oligonucleotides. As a vertically integrated supplier, we offer unmatched control, quality and continuity throughout our supply chain.
- Stringent risk mitigation: Our oligo synthesis team’s risk mitigation process safeguards the reliability of your assays. This includes screening for all sequences greater than 55 bases to ensure no template contamination infects the facility.
- Comprehensive OEM commercial services: We offer a range of fill and finish solutions to accelerate commercialisation of your product. Our services include customisable oligo mixes, formulation, custom packaging, labelling and kitting.